[ad_1]
The extreme acute respiratory syndrome coronavirus 2 (SARS-CoV-2) triggered the coronavirus illness 2019 (COVID-19) pandemic, which has claimed over 5.8 million lives as of February 14, 2022.
SARS-CoV-2 binds to human cells via using its spike protein, which attaches to the human angiotensin-converting enzyme 2 (ACE2) receptor current on the floor of host cells. In a latest Proceedings of the Nationwide Academy of Sciences of america of America examine, researchers counsel that extracellular vimentin (VIM) might act as a coreceptor to permit for SARS-CoV-2 entry into cells.
Research: Extracellular Vimentin Is an Attachment Issue That Facilitates SARS-Cov-2 Entry into Human Endothelial Cells. Picture Credit score: Kateryna Kon / Shutterstock.com
Introduction
VIM is a filamentous protein present in mesenchymal derivatives, together with endothelial cells. To this finish, this protein is current on the extracellular floor of endothelial cells and macrophages.
Earlier research have demonstrated that VIM is an attachment issue for SARS-CoV, Japanese encephalitis virus, and dengue virus. Thus, these observations might point out the important thing function of VIM in selling the entry of SARS-CoV-2 into human circulation and different organs.
SARS-CoV-2 has been recognized within the lungs, cardiovascular system, mind, coronary heart, liver, and kidneys of contaminated people. Though the ACE2 receptor is abundantly current within the higher respiratory tract, its focus within the decrease respiratory tract is low. Comparatively, many different potential receptors of SARS-CoV-2 have been recognized, together with Neuropilin-1, CD209L/L-SIGN, CD209/DC-SIGN, and heparan sulfate, which can facilitate its entry into lung cells.
Research findings
The present examine confirmed that the SARS-CoV-2 spike receptor-binding area (RBD) binds to VIM, which is current as intermediate filaments on the outer floor of cells and within the cytoplasm. Nevertheless, cells expressing VIM didn’t present elevated viral entry as in comparison with these expressing ACE2.
Nevertheless, when each VIM and ACE2 have been expressed collectively, viral entry into the cells was considerably elevated as in comparison with ACE2 only-expressing cells. The identical outcome was noticed with the addition of exogenous VIM to ACE2-expressing cells. Additional analysis confirmed that extracellular VIM is a possible coreceptor or attachment issue for SARS-CoV-2.
The researchers then decided whether or not VIM was current on the similar location as ACE2 on cells co-expressing each of those receptors. To this finish, they discovered VIM filaments within the cell membrane, particularly the place cells have been involved.
Furthermore, RBD certain extra strongly to cells expressing each molecules, even when recombinant soluble ACE2 was used to check RBD binding with or with out VIM. The outstanding enhance in RBD-ACE2 binding within the presence of VIM could also be resulting from a unique binding website for VIM on the RBD than for ACE2.
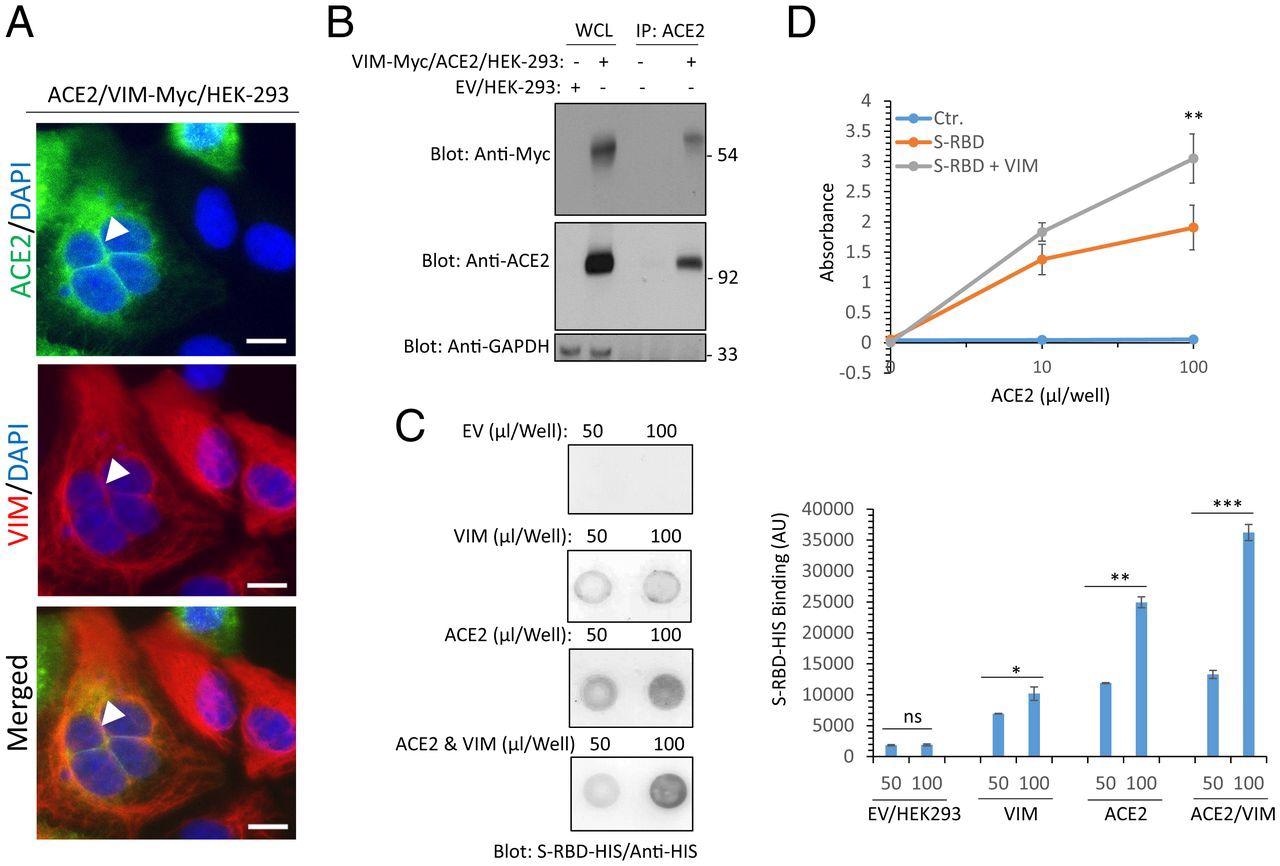
Extracellular VIM facilitates SARS-CoV-2 entry into human cells. (A) HEK-293 cells expressing ACE2 and VIM-Myc have been costained with anti-ACE2, anti-VIM and DAPI. The slides have been seen by confocal microscopy. White arrowheads present colocalization of VIM with ACE2. (Scale bars, 50 µM.) (B) WCL from HEK-293 cells expressing management EV or coexpressing VIM-Myc with ACE2 have been subjected to a coimmunoprecipitation assay utilizing anti-ACE2 antibody. The immunoprecipitated proteins have been analyzed by Western blot evaluation utilizing anti-Myc antibody for VIM. The identical membrane was additionally probed for ACE2 and GAPDH. (C) Completely different concentrations of WCL from HEK-293 cells expressing EV, VIM, ACE2, or coexpressing VIM with ACE2 have been blotted on the PVDF membrane. The membranes after blocking with BSA have been incubated with S-RBD-HIS-STRP (1 µg/mL), adopted with immunoblotting with anti-HIS antibody. Quantification of the blots is proven. (D) A 96-well plate coated with soluble ACE2 was incubated with RBD-HIS-STRP alone or RBD-HIS-STRP with CM containing VIM. The plate was subjected to ELISA and the binding of S-RBD with ACE2 decided with streptavidin-HRP. *P < 0.05, **P < 0.01, ***P < 0.001; ns, not important.
The researchers additionally examined the aggressive binding of the RBD with ACE2 within the presence of two anti-RBD antibodies CR3022 and S309. Each CR3022 and S309 have been discovered to bind outdoors the receptor-binding motif (RBM), with out instantly clashing with ACE2 for binding.
If the RBD is first handled with CR3022, VIM-RBD binding is lowered; nonetheless, this similar impact was not noticed with S309. This will point out that VIM and ACE2 bind to the RBD at two completely different websites. Moreover, CR3022 might rely upon the presence of VIM for its neutralizing exercise, as incubation of the RBD with this antibody prevents viral entry into VIM+ cells, however not when VIM is knocked out.
“Taken collectively, our information reveal the CR3022 antibody interferes with the binding of SARS-CoV-2-S and might operate as a neutralizing antibody in related cell varieties when each VIM and ACE2 are expressed.”
SARS-CoV-2 an infection occurred at a better charge in cells expressing each ACE2 and VIM than ACE2 alone, whereas VIM knockdown lowered an infection charges. This means the requirement for VIM for endothelial cell an infection by SARS-CoV-2, wherein case it could possibly be focused to forestall or deal with SARS-CoV-2 an infection. Furthermore, VIM often is the bridge binding the spike protein to the ACE2 receptor to advertise viral entry into the cell.
When the extent of VIM expression was elevated, the speed of an infection was reasonably elevated. Comparatively, a big enhance was noticed when ACE2 and VIM have been each current.
Implications
The outcomes present the important thing operate of VIM as an attachment and an infection issue for SARS-CoV-2 into ACE2-expressing cells. Along with lung cells, SARS-CoV-2 assaults endothelial cells and may also trigger harm to the cardiovascular system, kidneys, and gut.
The endothelial part of an infection by SARS-CoV-2 could also be an vital a part of the pathologic course of in COVID-19 since endothelial activation can mix with the inflammatory response in COVID-19. This causes a hyper-inflammatory and procoagulant state that leads to thrombosis, capillary occlusion inside the lungs, irregular blood vessel development, in addition to neurological options.
One other latest preprint additionally instructed that VIM is instrumental in SARS-CoV-2 an infection since anti-VIM antibody administration prevents an infection of ACE2-expressing cells by SARS-CoV-2. With the identical binding websites for each, a better degree of expression of VIM would have lowered RBD-ACE2 binding by competitors, which isn’t the case.
CR3022, an RBD-binding antibody, neutralizes SARS-CoV-2, presumably via its inhibition of VIM binding, whereas not stopping ACE2 binding. Additional analysis is required to determine different receptors which will work together with VIM, as earlier animal fashions with ACE2 knockdown confirmed a slight discount in an infection by SARS-CoV-2, whereas VIM knockdown led to a big enhance.
The widespread expression of VIM on mesenchymal derivatives, in addition to on sort 2 alveolar pneumocytes and nasal goblet cells, is elevated throughout irritation and viral an infection. This will point out “a possible function for VIM in an infection of nasal and lung epithelial cells,” particularly since ACE2 expression on lung alveolar cells and endothelial cells is kind of low, requiring VIM to behave as an attachment issue and improve the viral entry.
“Taken collectively, the observations we current might result in the event of latest antiviral therapeutics combining therapies that inhibit interactions of each ACE2 and VIM with SARS-CoV-2.”
Journal reference:
- Amraei, R., Xia, C., Oleknik, J., et al. (2022). Extracellular Vimentin Is an Attachment Issue That Facilitates SARS-Cov-2 Entry into Human Endothelial Cells. Proceedings of the Nationwide Academy of Sciences of america of America. doi:10.1073/pnas.2113874119.
[ad_2]