
[ad_1]
In a latest article posted to the bioRxiv* preprint server, researchers at Rutgers, the State College of New Jersey, the College of South Florida, and the Catholic College of America demonstrated that the pure extreme acute respiratory syndrome coronavirus 2 (SARS-CoV-2) fundamental protease (Mprofessional) mutations lead to nirmatrelvir resistance.
 Research: Naturally occurring mutations of SARS-CoV-2 fundamental protease confer drug resistance to nirmatrelvir. Picture Credit score: Orpheus FX / Shutterstock
Research: Naturally occurring mutations of SARS-CoV-2 fundamental protease confer drug resistance to nirmatrelvir. Picture Credit score: Orpheus FX / Shutterstock
Background
The continued SARS-CoV-2 pandemic emphasizes the urgency for orally bioavailable antiviral drugs. The Mprofessional of the SARS-CoV-2 is a cysteine protease and a acknowledged goal for antiviral medicine. Paxlovid (nirmatrelvir) and molnupiravir are the one two oral SARS-CoV-2 drugs which have acquired Meals and Drug Administration (FDA) approval. Paxlovid contains the Mprofessional inhibitor nirmatrelvir and the metabolic enhancer ritonavir.
The danger of hospitalization or dying was 89% decrease in coronavirus illness 2019 (COVID-19) sufferers who acquired Paxlovid lower than three days of the graduation of SARS-CoV-2 signs than in those that bought the placebo. Nonetheless, the looks of SARS-CoV-2 variants with Mprofessional mutations prompted issues about possible drug resistance.
Concerning the research
The current work sought to find Mprofessional drug-resistant SARS-CoV-2 mutants related to naturally occurring polymorphisms. The researchers examined the SARS-CoV-2 sequences within the World Initiative on Sharing Avian influenza Information (GISAID) database.
The staff examined the drug resistance of three Mprofessional inhibitors: PF00835231, GC-376, and nirmatrelvir. GC-376 was used for treating feline infectious peritonitis virus (FIPV) an infection amongst cats. Additional, Pfizer developed phosphonate PF-00835231 prodrug as an intravenous agent for treating COVID-19 sufferers in hospitals and was progressed to scientific trial. Lastly, nirmatrelvir was the lively ingredient in Paxlovid.
The authors concentrated on 12 Mprofessional lively web site residues, M49, H41, N142, T135, S144, H164, H163, M165, H172, E166, and Q189, discovered inside 6 Å of the nirmatrelvir binding area, i.e., PDB: 7SI9, to search out drug-resistant Mprofessional mutants. They proposed that mutations throughout the lively web site residues instantly impression substrate adherence and drug resistance. To check this concept, the researchers evaluated the mutations in these 12 residues using the SARS-CoV-2 sequences from the GISAID.
The thermal shift binding evaluation and an enzymatic assay primarily based on fluorescence resonance vitality switch (FRET) have been used to find out the drug sensitivity. The SARS-CoV-2 Mprofessional mutant proteins have been expressed and examined for nirmatrelvir inhibition and enzymatic exercise using X-ray crystal buildings.
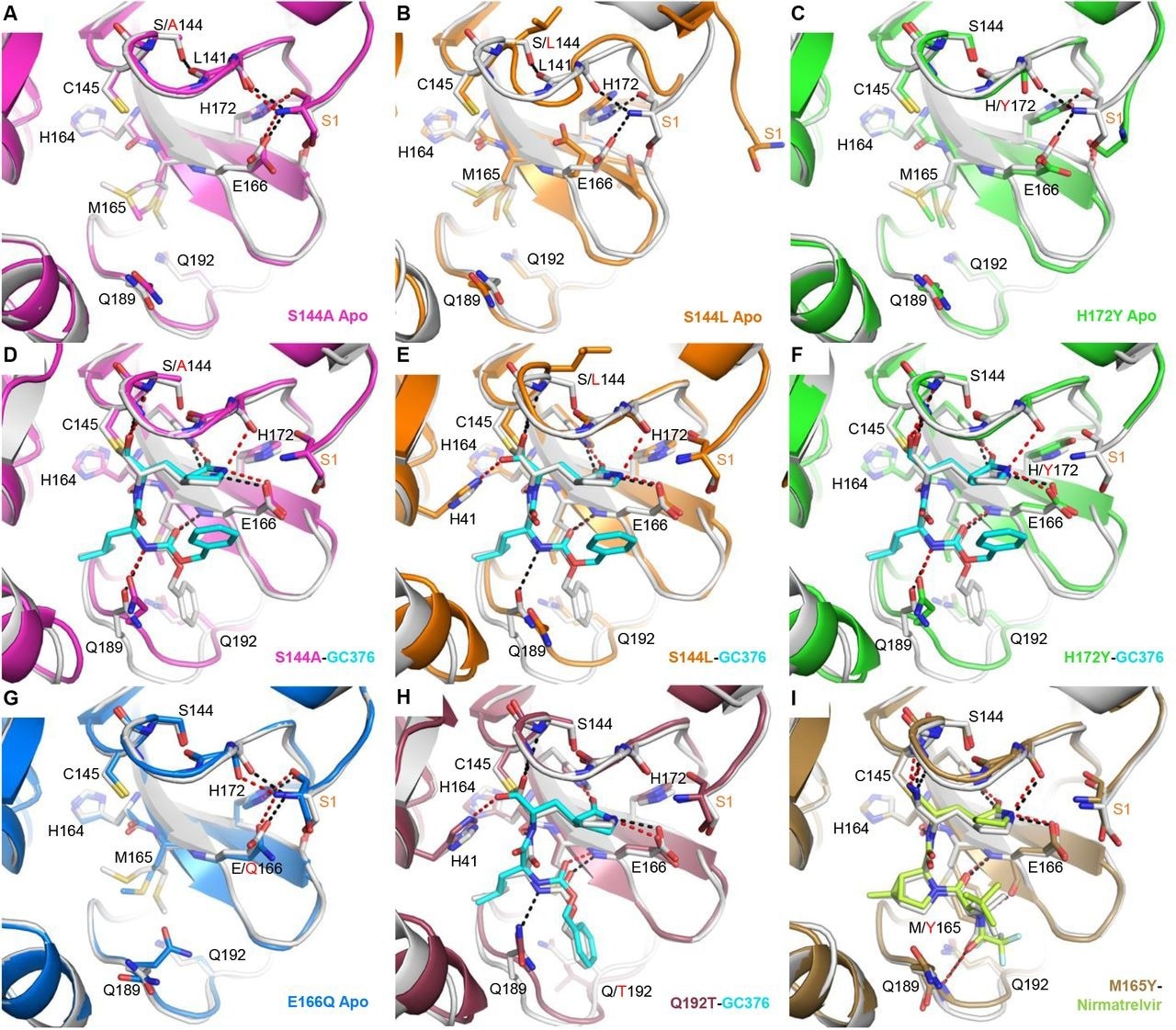
X-ray crystal buildings of Mpro mutants. Every mutant construction is aligned with the corresponding WT construction proven in white (apo, PDB 7JP1; GC-376 advanced, 6WTT; nirmatrelvir advanced, 7RFW). For the mutant buildings, GC-376 and nirmatrelvir are proven in cyan and neon inexperienced respectively. WT HBs are proven as black dashes for chosen residues on the mutation websites or between the protein and inhibitor. Mutant HBs are proven as purple dashes. Mutations are indicated with purple textual content. S1 residue from the N-terminus of the adjoining protomer is labeled in orange. The aspect chain of L141 will not be proven. (A) Apo Mpro S144A (magenta, PDB 8D4L). (B) Apo Mpro S144L (orange, PDB 8DFE). The view for panel B is shifted barely to indicate for the motion of the adjoining N-terminus. (C) Apo Mpro H172Y (inexperienced, PDB 8D4J). (D) Mpro S144A GC-376 advanced (magenta, PDB 8D4M). (E) Mpro S144L GC-376 advanced (orange, PDB 8DD9). (F) Mpro H172Y GC-376 advanced (inexperienced, PDB 8D4K). S1 residue is disordered. (G) Apo Mpro E166Q (blue, PDB 8D4N). (H) Mpro Q192T GC376 advanced (mauve, PDB 8DGB). (I) Mpro M165Y Nirmatrelvir advanced (brown, PDB 8DCZ).
Outcomes
The authors discovered 66 frequent SARS-CoV-2 Mprofessional mutations positioned on the nirmatrelvir binding web site, spanning all high-frequency mutants on the 12 analyzed residues.
In accordance with the kcat/Km worth for enzymatic exercise and the Ki worth for drug resistance, the Mprofessional mutants have been divided into three classes: class 1 incorporates important residues for enzymatic exercise (H163 and H41); class 2 incorporates sizzling spot residues for drug resistance (M165, S144, H172, E166, and Q192); and class 3 contains residues that may face up to a number of mutations with out considerably influencing drug inhibition and the enzymatic exercise (M49, T135, N142, Q189, and H164).
Most Mprofessional mutants exhibited diminished enzymatic exercise, i.e., kcat/Km better than a 10-fold lower. However, 11 mutants, together with M165T, S144M/F/A/G/Y, H172Q/F, E166Q, and Q192T/S/V, displayed comparable enzymatic exercise because the SARS-CoV-2 wild-type pressure with kcat/Km lower than a 10-fold modification and resistance to nirmatrelvir with Ki better than a 10-fold hike.
Though the enzymatic exercise of a number of single mutants discovered on this investigation was decreased, it was conceivable that the virus may produce a number of mutations, such because the H172Y/Q189T mutant, helping within the restoration of replication health, keep and even enhance drug resistance.
In addition to, the X-ray buildings illustrated how Mprofessional mutations influenced the conformational stability of the lively web site, decreasing ligand binding. X-ray buildings of six single mutants with or with out nirmatrelvir/GC-376 have been established.
The staff famous that the mutants tackle a productive configuration when a substrate or inhibitor is current, which explains how they will catalyze the response. The discount within the catalytic properties of the mutants and inhibition by nirmatrelvir/GC-376 seems to be brought on by two components: an entropic impact as a result of lively web site’s elevated conformational instability and an enthalpic impression brought on by the direct disruption of ligand-binding contacts.
Conclusions
General, within the present analysis, the authors discovered 66 drug-resistant Mprofessional mutants arising from pure SARS-CoV-2 polymorphism from the GISAID database. These mutations could impression the effectiveness of Paxlovid, and steady Paxlovid prescription may most likely elevate the prevalence of those pre-existent drug resistance mutants.
The nirmatrelvir resistance hotspots, Mprofessional M165, S144, H172, E166, and Q192 mutants, should be monitored actively throughout the circulating SARS-CoV-2s. Most probably, mutations at these residues will lead to appreciable drug resistance whereas sustaining the enzyme operate. Whether or not these mutations scale back the viral replication capability and transmission wants additional evaluation.
Double or triple mutants could come up to counteract the lack of enzymatic operate from the only mutant whereas enhancing or sustaining drug resistance, as seen by the H172Y/Q189T mutant. Consequently, it’s important to trace the Mprofessional mutants with decreased enzymatic exercise from the present research.
*Essential discover
bioRxiv publishes preliminary scientific experiences that aren’t peer-reviewed and, due to this fact, shouldn’t be thought to be conclusive, information scientific observe/health-related habits, or handled as established info.
Journal reference:
- Naturally occurring mutations of SARS-CoV-2 fundamental protease confer drug resistance to nirmatrelvir; Yanmei Hu, Eric M Lewandowski, Haozhou Tan, Ryan T Morgan, Xiujun Zhang, Lian M Jacobs, Shane G Butler, Maura V Mongora, John S Choy, Yu Chen, Jun Wang. bioRxiv preprint 2022, DOI: https://doi.org/10.1101/2022.06.28.497978, https://www.biorxiv.org/content material/10.1101/2022.06.28.497978v1
[ad_2]



