
[ad_1]
A current analysis letter posted to the JAMA Community Open assessed the sturdiness of immunity conferred by the coronavirus illness 2019 (COVID-19) messenger ribonucleic acid (mRNA)-1273 vaccine.
 Research: Sturdiness of Safety Towards Symptomatic COVID-19 Amongst Members of the mRNA-1273 SARS-CoV-2 Vaccine Trial. Picture Credit score: Rido / Shutterstock
Research: Sturdiness of Safety Towards Symptomatic COVID-19 Amongst Members of the mRNA-1273 SARS-CoV-2 Vaccine Trial. Picture Credit score: Rido / Shutterstock
Background
Assessing the longevity of immunity imparted by the extreme acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines is among the many public well being priorities. Additional, the findings are required to tell pointers on nonpharmaceutical interventions and booster vaccinations for COVID-19.
Concerning the examine
Within the current work, the researchers evaluated the mRNA-1273 P301 cohort examine, an ongoing part 3, randomized, placebo-controlled trial of 30 415 US adults to evaluate the efficacy and security of the mRNA-1273 SARS-CoV-2 (Moderna) vaccine.
The staff used the per-protocol inhabitants for this cohort evaluation, which comprised 28,451 topics who have been SARS-CoV-2-negative on the initiation of the examine and acquired two vaccine doses by the completion of the blinded part. The volunteers acquired the preliminary vaccine dose between 27 July and 23 October 2020.
A minimal of two systemic signs or one respiratory symptom or signal outlined the COVID-19 instances and have been validated by a SARS-CoV-2-positive reverse transcriptase-polymerase chain response (RT-PCR) take a look at outcome. Earlier than enrollment, all members submitted a written knowledgeable consent. The scientists adhered to the Strengthening the Reporting of Observational Research in Epidemiology (STROBE) reporting requirements.
The authors used a Cox regression mannequin that represented the log hazard ratio for the vaccine influence as a piecewise-linear operate of time after immunization, with change factors positioned at 120, 80, and 40 days following dose 1 on the timestamps the place the log hazard ratio’s slope was anticipated to alter, to acquire an correct estimation of safety. The entire curve of vaccine effectiveness (VE) on the current chance of illness could be estimated utilizing this formulation.
The investigators used calendar time after the examine started because the time scale for the evaluation as a substitute of time following topic randomization because the frequency of COVID-19 neighborhood transmission differed dramatically over time. This enabled them to match the sickness incidence amongst unvaccinated and vaccinated folks on the identical calendar date. The examine was carried out with the model 4.1 R bundle DOVE, with the dove2 operate.
Research findings
The examine outcomes indicated that the placebo group had 14,164 members with 769 SARS-CoV-2 an infection instances, whereas the mRNA-1273 group comprised 14,287 topics with 56 COVID-19 incidents. The VE rose steadily to a peak of 94.1% on day 120, successive to 92.6% 40 days following the preliminary dose. At round 120 days, the VE started to say no, and it had fallen to 89.6% by 200 days.
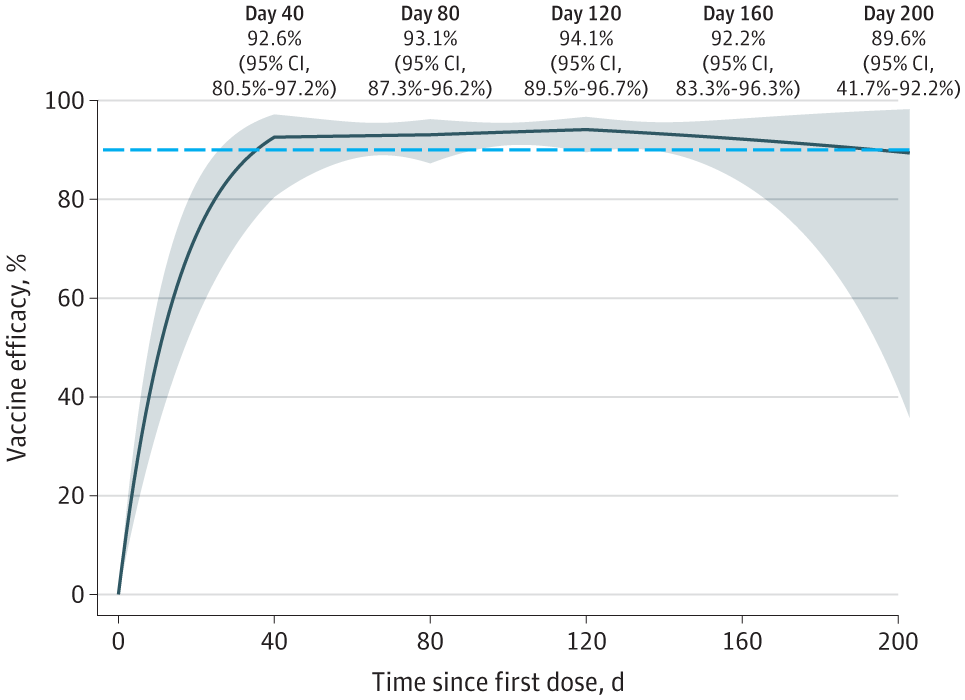
Efficacy of the mRNA-1273 SARS-CoV-2 Vaccine in Lowering the Present Threat of Symptomatic COVID-19
These findings exhibit that VE was declining with time and was extra insightful concerning the size of safety in the direction of symptomatic sickness than prior estimates. Though there was a substantial ambiguity in predicting VE near the completion of blinded follow-up, the diploma of safety was but excessive 200 days following dose 1.
Conclusions
In response to the examine information, the COVID-19 Moderna VE in opposition to symptomatic SARS-CoV-2 an infection was assessed to be 94.1% at interim screening and 93.2% on the finish of the blinded part. Upon the comparisons of those two estimations, it seems that VE was diminishing barely.
However, because the VE estimate was acquired below the presumption that VE was secure in the course of the evaluation interval, it represents a median of the time-varying vaccine influence all through a broad analysis interval, weighted by when the incident occurs as a substitute of the VE after the investigation interval. Therefore, this comparability was inadequate to determine the precise extent of waning.
The staff said that the part III trials provide placebo-regulated efficacy estimates for much less than seven months following dose 1 because of the overlap of placebo members with the vaccine group. Certainly, within the present investigation, few instances of COVID-19 occurred following six months, making it not possible to find out the extent of VE decline after the blinded follow-up. The authors famous that observational research might reveal info on vaccines’ long-term benefits.
[ad_2]



