[ad_1]
In a latest examine posted to the Analysis Sq. preprint* server, researchers consider how the D614G and P681H mutations alter the attributes of the extreme acute respiratory syndrome coronavirus 2 (SARS-CoV-2) D614G pressure, in addition to the Alpha, Delta, and Omicron variants of concern (VOCs).
Research: SARS-CoV-2 variants’-Alpha, Delta, and Omicron D614G and P681R/H mutations impression virus entry, fusion, and infectivity. Picture Credit score: FOTOGRIN / Shutterstock.com
Background
With a mutation fee of roughly 1.1 × 10-3 substitutions/web site/12 months, a number of SARS-CoV-2 VOCs have emerged which can be extremely infectious and transmissible. Whereas the Alpha and Delta variants resulted within the second and third coronavirus illness 2019 (COVID-19) pandemic waves, respectively, most just lately, in November 2021, the SARS-CoV-2 variant Omicron variant emerged in South Africa and subsequently led to the fourth pandemic wave all over the world.
The SARS-CoV-2 D614G pressure noticed in Might 2020 consists of a single mutation and was extremely infectious. Though D614G an infection resulted in a excessive viral load within the higher respiratory tract, its an infection didn’t considerably alter illness severity as in comparison with the ancestral SARS-CoV-2 Wuhan-1 pressure.
The primary SARS-CoV-2 VOC to be recognized was the Alpha variant, which additionally had the D614G mutation and a cleavage web site mutation P681H. Comparatively, the SARS-CoV-2 Delta variant additionally had D614G and P681R mutations within the spike (S) area.
The just lately recognized Omicron VOC harbors the D614G and P681H mutations, in addition to a mutated S proline 681 to histidine as a substitute of arginine, which was additionally noticed within the Delta variant.
There stays a restricted quantity of research which have evaluated the impression of D614G and P681H/R mutations on the traits of VOCs. Nonetheless, it’s essential to grasp the mechanisms behind the entry and fusion of rising VOCs, particularly as they supply immune escape means to those VOCs and cut back the neutralization efficacy of present vaccines.
In regards to the examine
Within the current examine, researchers used the full-length wild-type ancestral Wuhan-1 S protein and subjected it to site-directed mutagenesis. To this finish, the researchers synthesized the D614G, Alpha, Delta, Omicron, single P681H mutant, and D614G+P681R double mutant pseudoviruses.
The researchers ready pseudoviruses in 293T cells containing furin-like proteases. As in comparison with the Delta pseudovirus, Alpha and Omicron pseudoviruses confirmed slower S cleavage processing within the presence of furin. All mutants have been well-expressed in BHK-21 transfected cells.
S protein expression of all of the variant and mutant pseudoviruses on the cell floor, as in comparison with wild sort S, was assessed utilizing circulate cytometry in HEK293T mammalian cells. Whereas analyzing the binding of surface-expressed S protein to soluble human angiotensin-converting enzyme 2 (hACE2) by circulate cytometry, the authors famous that the Delta, Omicron, and D614G+P681R S mutants confirmed a two-fold enhance in binding to soluble hACE2.
The researchers additionally analyzed the cleavage of S protein in pseudovirions at 24 and 48 hours post-transfection. Subsequent, they analyzed concentrated pseudovirions utilizing Western blot.
Aside from Alpha pseudovirus S, the S proteins of all different variants have been cleaved at 24 hours post-transfection. Comparatively, for the Omicron pseudovirus, S cleavage was observable at 48 hours post-transfection.
Research findings
The Delta variant confirmed enhanced virus entry in hACE2 and transmembrane protease, serine 2 (TMPRSS2) overexpressed cells. However, the double mutant D614G+P681R confirmed a marked enhance in virus entry by way of each hACE2 and TMPRSS2 receptors, just like Delta pseudovirus.
The artificial double mutant D614G+P681R additionally confirmed excessive infectivity titer in each hACE2 and TMPRSS2 overexpressed cells. This means the potential position of those two mutations in hACE2 and TMPRSS2 mediated virus entry.
The Omicron pseudovirus confirmed hACE2-dependent virus entry, low fusion, syncytial formation, and lowered infectivity titers. Apparently, the marked discount in fusion capability of Omicron pseudoviruses was as a result of presence of P681H mutation within the Omicron S protein. Additional, all variants confirmed a big enhance in infectivity titers in 293T-hACE2 cells.
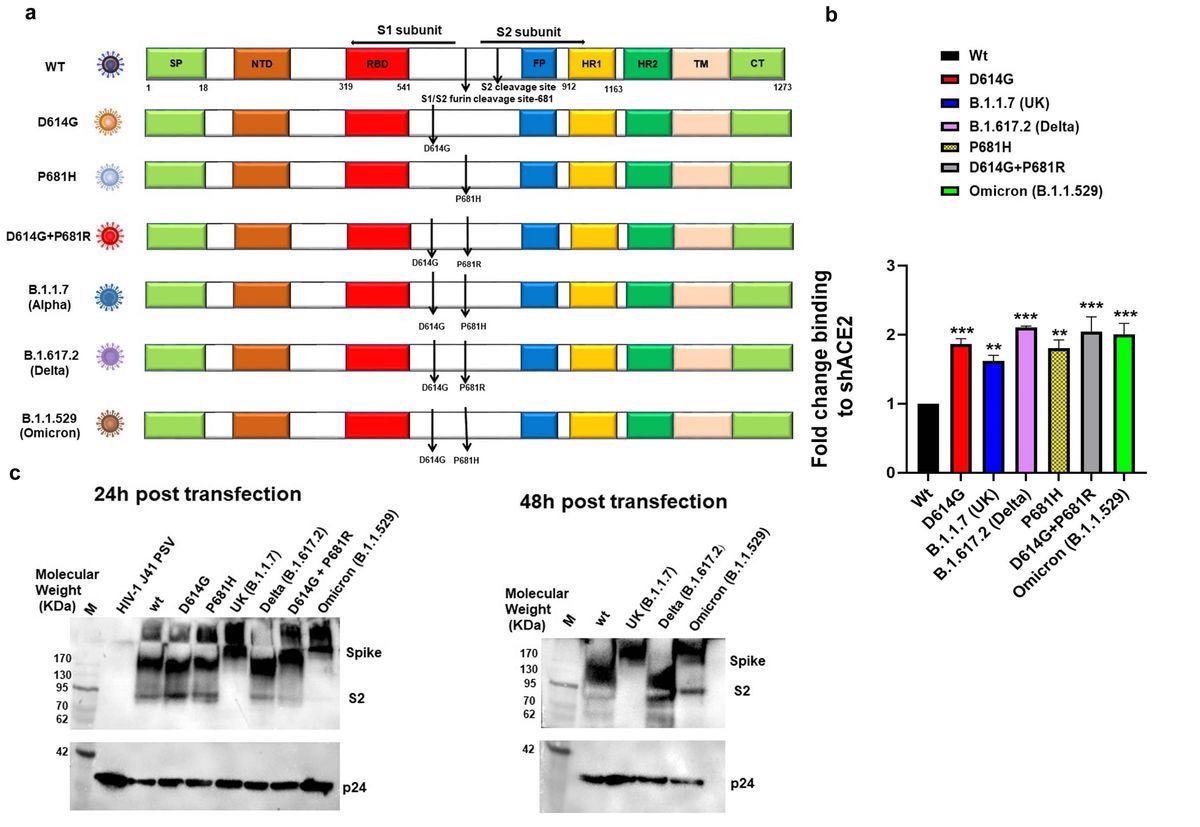
Expression and cleavage of SARS-CoV-2 variants and artificial mutants: a. Schematic illustration of ancestral, Alpha, Delta and Omicron spike protein with D614G and P681R/H mutations and artificial mutants, the sign peptide (inexperienced field), S1-N terminal area NTD (brown field), Receptor binding area (Purple field), fusion peptide (blue field),Heptad repeat 1(yellow field), Heptad repeat 2(darkish inexperienced field), Transmembrane area TM (Cream field), Cytoplasmic tail CT (inexperienced field), S1/S2 and S2′ cleavage web site, presence of D614G, P681H/R mutation b. Floor expressed spike protein binding to soluble-hACE2 was analyzed by expressing the spike of variants and mutants in HEK 293T cells and 36 h post-transfection incubated with soluble-hACE2 and analyzed by circulate cytometry. Every worth represents a single imply worth of two repeated experiments. Statistical significance was decided utilizing the one-way ANOVA check (p < 0.05), the place *** and ** p < 0.05 is critical. c. Detection of spike protein cleavage in numerous pseudoviruses at 24h and 48h post-transfection by Western blot evaluation, p24 was stored as loading management.
Additional evaluation of SARS-CoV-2 variant S proteins confirmed that the P681R mutation allowed elevated cell-to-cell fusion within the presence of exogenous trypsin within the Delta and double D614G+P681R mutant S proteins. This phenomenon was not noticed with the Omicron S protein, which has the P681H mutation and single P681H mutant. These findings recommend that the P681H mutation within the Omicron S cleavage web site down-regulated the cleavability of S protein by furin or trypsin-like proteases, thus limiting localized replication of Omicron.
The authors additionally famous that Omicron pseudovirus titers elevated in hACE2 cells and virus entry was lowered to greater than 50% within the presence of cathepsin L inhibitor E64d. Nonetheless, within the presence of TMPRSS2 inhibitor camostat mesylate, there was a discount of about 34% within the entry to host cells. These findings urged that Omicron preferentially entered host cells by way of ACE2-mediated endocytosis.
Conclusions
Total, the SARS-CoV-2 Delta and Omicron VOCs demonstrated the evolution of each virulent and reasonably virulent strains for higher health for survival. Within the Delta variant, the presence of the D614G and P681R mutations was essential for virulence and pathogenesis.
Comparatively, within the Omicron variant, the P681H mutation resulted in gradual processing of S cleavage by furin or trypsin-like proteases and allowed ACE2-dependent entry. This subsequently restricted Omicron replication primarily to the higher respiratory tract. The only artificial mutant P681H additionally intently resembled Omicron in its habits.
Growing an understanding of the SARS-CoV-2 entry pathway will enable for the suitable number of cell traces for in vitro screening of medicine and vaccine candidates.
*Necessary discover
Analysis Sq. publishes preliminary scientific stories that aren’t peer-reviewed and, subsequently, shouldn’t be considered conclusive, information medical observe/health-related habits, or handled as established data.
Journal reference:
- Khatri, R., Siddqui, G., Sadhu, S., et al. (2022). SARS-CoV-2 variants’-Alpha, Delta, and Omicron D614G and P681R/H mutations impression virus entry, fusion, and infectivity. Analysis Sq.. doi:10.21203/rs.3.rs-1310197/v1.
[ad_2]